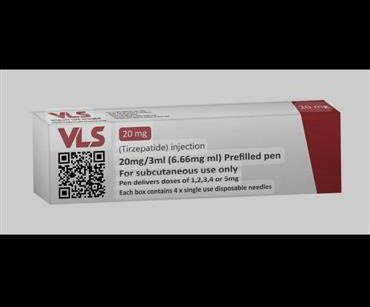
VLS Tirzepatide 20 mg Classification
VLS Tirzepatide 20 mg falls under the class of dual incretin receptor agonists, specifically as a GIP / GLP‑1 receptor agonist. This means the molecule is designed to mimic and act upon two gut hormones — GIP (glucose‑dependent insulinotropic polypeptide) and GLP‑1 (glucagon‑like peptide‑1). Its classification places it among advanced metabolic therapies used primarily in type 2 diabetes management and weight management / obesity treatment.
- Drug class: Incretin mimetic (dual agonist)
- Therapeutic category: Metabolic / antidiabetic / weight‑loss adjunct
- Regulatory status (UK / EU): Under scrutiny and prescription‑only in many jurisdictions
- Brand / generic relationship: VLS is your product name; it mirrors the active ingredient “tirzepatide” used in branded formulations (e.g. Mounjaro in UK contexts)
This classification informs healthcare professionals and consumers about where VLS Tirzepatide 20 mg sits in modern therapeutic hierarchies, and it underscores that it is not a simple supplement but a potent prescription‑level agent.
What is VLS Tirzepatide 20 mg?
VLS Tirzepatide 20 mg is a concentrated formulation of tirzepatide formulated for subcutaneous injection (under the skin). Its purpose is to target metabolic pathways that affect blood sugar, appetite, and weight.
Key features:
- Dual hormone mimicry: Activates both GLP‑1 and GIP receptors to enhance insulin response and suppress glucagon.
- Weekly dosing: Designed for once‑weekly injection, a convenience compared to daily therapies.
- Adjunct therapy: Intended to be used alongside diet, exercise, and possibly concurrent antidiabetic medications.
- Potential for weight management: In clinical settings, tirzepatide has shown significant weight loss effects in many patients.
Effectively, VLS Tirzepatide 20 mg is a high‑strength option in the family of tirzepatide products—subject to medical oversight and suitable only for patients who meet specific eligibility criteria.
Recommended Dosage for VLS Tirzepatide 20 mg
Because 20 mg is a relatively high strength, the recommended dosage regimen must be strictly calibrated. Below is a guideline (derived from published clinical use of tirzepatide), but consultation with a qualified prescriber is essential.
Typical titration scheme (example):
- Start low: Begin with 2.5 mg once weekly for 4 weeks
- Incremental increases: After 4 weeks, increase to 5 mg weekly
- Further scaling: If additional control is required, rise in 2.5 mg increments every 4 weeks
- Max limit in trials: Many trials stop at 15 mg weekly — usage of 20 mg is more aggressive and less studied
Important dosage notes:
- You must not escalate faster than once every 4 weeks.
- If a dose is missed, you may administer it within 4 days (96 hours); past that, skip the missed dose.
- Never inject two doses too close together — maintain at least 72 hours (3 days) gap.
Because 20 mg is beyond typical maintenance ranges, that strength might be reserved for custom compounding or advanced regimens—only under specialist supervision.
How Does VLS Tirzepatide 20 mg Work?
The mechanism of action for VLS Tirzepatide 20 mg is rooted in its dual activation of incretin receptors:
- GLP‑1 receptor agonism
- Enhances glucose‑dependent insulin secretion
- Suppresses glucagon release when blood glucose is elevated
- Slows gastric emptying, which prolongs satiety
- GIP receptor agonism
- Bolsters insulin release in response to meals
- May affect fat metabolism and energy partitioning
Together, these actions yield synergistic effects:
- Reduced postprandial glucose spikes
- Increased fullness and reduced appetite
- Slower digestion of food (gastric emptying delay)
- Improved insulin sensitivity over time
Thus, VLS Tirzepatide 20 mg acts not only to lower blood sugar but also to influence the regulatory systems of hunger, satiety, and energy storage.
Benefits of Taking VLS Tirzepatide 20 mg
When used responsibly and under medical supervision, VLS Tirzepatide 20 mg may offer a range of benefits beyond simple glucose lowering:
- Superior glycaemic control: Lower HbA1c and fasting glucose
- Weight loss support: Many patients experience significant weight reduction during clinical trials
- Reduced appetite: Helps reduce caloric intake naturally
- Lipid improvements: Potential favourable effects on cholesterol and triglycerides
- Reduced cardiovascular risk (potential): In some studies, GLP-1–based therapies show cardioprotective benefits (though long‑term data is still emerging)
- Convenient schedule: Once‑weekly dosing enhances adherence
- Dual effect: Unlike single incretin agents, it combines two hormonal pathways
These benefits make VLS Tirzepatide 20 mg an attractive option for those with type 2 diabetes, obesity, or metabolic syndrome—provided they are suitable candidates and under supervision.
When Should You Take VLS Tirzepatide 20 mg?
Proper timing can enhance effectiveness and reduce side effects:
- Once weekly administration on the same day each week
- Time of day: can be taken at any time, with or without food, as tolerated
- Match with your schedule: e.g. always Monday mornings or evenings — consistency is key
- Adjust the day only if necessary, maintaining a minimum 72‑hour gap between doses (so doses don’t run too close)
- If dose is missed and within 96 hours, take it immediately, otherwise skip and resume schedule
Consistency in timing helps maintain steady plasma levels and reduces fluctuations in side effect manifestation.
When Should You Not Take VLS Tirzepatide 20 mg?
There are important contraindications and situations of caution. You should avoid using VLS Tirzepatide 20 mg if:
- You have or a family history of medullary thyroid carcinoma (MTC)
- You are diagnosed with Multiple Endocrine Neoplasia syndrome type 2 (MEN-2)
- You have severe gastrointestinal disorders, gastroparesis, or pancreatitis history
- You suffer from severe renal or hepatic impairment without careful monitoring
- You are pregnant or breastfeeding — safety is not established
- You are allergic to tirzepatide or any component of the formulation
- You are under 18 years of age
- You have type 1 diabetes (not indicated in that setting)
Additionally, use with caution when combining with medications that may increase risk of hypoglycaemia (e.g. insulin, sulfonylureas) or in patients with diabetic retinopathy.
Always seek a medical review before initiating VLS Tirzepatide 20 mg to exclude contraindications.
What is the Mechanism of VLS Tirzepatide 20 mg
This section re‑emphasises and expands the deep mechanism behind VLS Tirzepatide 20 mg:
- GLP‑1 receptor binding: triggers cascade that enhances insulin release in response to high blood glucose, suppresses glucagon, slows gastric emptying, and lowers appetite.
- GIP receptor binding: further stimulates insulin secretion and may influence fat metabolism.
- The dual agonism yields a stronger, more balanced metabolic modulation than GLP‑1 alone.
- Slowed gastric emptying contributes to blunted glucose absorption peaks and prolonged satiety.
- Over time, modulation of insulin sensitivity, lipid pathways, and energy balance can contribute to sustained metabolic improvements.
In essence, VLS Tirzepatide 20 mg reprograms the hormonal feedback loops of appetite, digestion, insulin release, and energy storage.
Uses of VLS Tirzepatide 20 mg
The primary approved and off‑label uses (depending on jurisdiction and oversight) include:
- Type 2 diabetes mellitus
- As an adjunct therapy to diet, exercise, and possibly other antidiabetic agents.
- To reduce HbA1c and control fasting and postprandial glucose.
- Weight management / Obesity
- For individuals with obesity or overweight with comorbidities.
- To assist in sustained weight loss over many months.
- Metabolic syndrome / prediabetes (investigational)
- As a preventive or early intervention in high‑risk individuals.
Use always requires monitoring and clinical review; outcomes depend on concurrent lifestyle changes.
Warnings and Precautions for VLS Tirzepatide 20 mg
Use of VLS Tirzepatide 20 mg comes with several important safeguards and monitoring concerns:
- Thyroid tumour risk: Animal studies showed thyroid C‑cell tumours; human risk unclear. Avoid in patients with MTC/MEN-2 history.
- Pancreatitis: Acute pancreatitis has been reported; monitor for severe abdominal pain radiating to the back.
- Dehydration / renal injury: GI side effects (vomiting, diarrhea) can lead to volume depletion and acute kidney injury.
- Diabetic retinopathy worsening: In some patients with preexisting retinopathy, risk of worsening exists.
- Hypoglycaemia risk: When used with other glucose-lowering therapies, risk increases. Adjust concomitant therapies carefully.
- Gastrointestinal effects: Nausea, vomiting, diarrhea — usually at the start or dose escalations.
- Behavioural / psychiatric effects: Rare reports of mood changes, suicidal ideation—monitor for mental health changes.
- Avoid in severe hepatic impairment until data supports safety.
- Medication interactions: Monitor concurrent use of drugs that affect GI motility, absorption, or those with narrow therapeutic windows.
Strict medical supervision, baseline tests, periodic monitoring (renal, pancreas, thyroid) are essential.
Side Effects of VLS Tirzepatide 20 mg
As with all potent medications, side effects are possible. Some are common and manageable, others rare but serious. Always balance risks vs benefits.
Common / Frequently Reported
- Nausea
- Vomiting
- Diarrhoea / loose stools
- Constipation
- Abdominal pain / cramping
- Decreased appetite
- Indigestion / dyspepsia
- Injection site reactions (redness, itching)
- Flatulence / burping
These typically occur during dose escalation and often diminish over time as the body adapts.
Less Common / Serious
- Pancreatitis (severe upper abdominal pain, vomiting)
- Mild acute kidney injury (due to dehydration)
- Gallbladder disease
- Thyroid C‑cell tumour risk (animal data)
- Hypoglycaemia (especially with insulin or sulfonylureas)
- Worsening diabetic retinopathy
- Allergic reactions (rash, swelling, breathing difficulties)
- Mental health changes, mood swings, suicidal ideation
If you experience symptoms such as severe abdominal pain, persistent vomiting, signs of pancreatitis, or sudden mood changes, seek medical attention immediately.
User reports (e.g. in forums) indicate that GI distress is among the most commonly discussed side effects, especially when doses are increased too rapidly.
Storage for VLS Tirzepatide 20 mg
Proper storage is crucial to maintain drug stability and efficacy:
- Refrigeration: Store between 2 °C and 8 °C (in a refrigerator). Do not freeze.
- In original container: Keep in its protective carton to minimise light exposure.
- Room temperature allowance: Once removed from fridge, may be stored at room temperatures (up to 21 days) but never re‑refrigerate.
- Check solution: Should be clear, colourless or slightly yellow. Discard if cloudy, discoloured, or containing particles.
- Needle/pen usage: Use a new needle for each injection; do not share pens or reuse.
- Dispose safely: Dispose of used needles and any unused pens in appropriate sharps containers. Do not flush medications.
Following correct storage extends shelf life and preserves safety.
Where to Buy VLS Tirzepatide 20 mg?
At Rohm Steroids, we specialise in high-quality pharmaceutical formulations distributed across UK markets with appropriate legal compliance. Here’s how you can purchase:
- Online ordering: Via our secure website with verified prescriptions
- Prescription requirement: A valid UK prescription from a licensed clinician is mandatory
- Delivery options: Discreet packaging, tracked courier service across the UK
- Quality assurance: Each batch is tested for purity, sterility, and potency
- Customer support: We provide guidance on dosing schedules, shipping queries, and product handling
Why choose us?
- UK‑based business with local compliance
- Competitive pricing vs branded equivalents
- Reliable supply chain
- Discreet and prompt shipping
(Note: Because VLS Tirzepatide 20 mg is a potent therapy, any purchase is contingent on medical approval.)
Frequently Asked Questions
Q1. Is 20 mg a standard dose for tirzepatide?
A. No — clinical trials most commonly test up to 15 mg weekly. The 20 mg strength is more aggressive and would be considered only under specialist direction.
Q2. Can I use VLS Tirzepatide 20 mg for weight loss even if I don’t have diabetes?
A. Some patients use tirzepatide off‑label for weight management; however, this must be medically endorsed and may not be approved under UK regulations.
Q3. How soon will I see results?
A. Glycaemic improvements may begin within weeks; weight loss typically emerges over months. Maximum effects often seen between 12–24 weeks.
Q4. Will I experience gastrointestinal side effects forever?
A. Not usually — many side effects reduce over time, especially after dose stabilisation.
Q5. Can I stop taking it if I lose enough weight or improve my sugars?
A. Discontinuation should only be done under medical supervision; rebound weight gain or glycaemic deterioration may occur.
Q6. Is monitoring required during therapy?
A. Yes — regular checks of blood glucose / HbA1c, renal function, pancreatic enzymes, thyroid status, and eye exams where relevant.
Q7. Can VLS Tirzepatide 20 mg be used alongside insulin or other antidiabetics?
A. Yes, but dose adjustments are often necessary to avoid hypoglycaemia. Close monitoring is essential.
Q8. What happens if I overdose?
A. Symptoms may include profound nausea, vomiting, GI distress, low blood sugar. Seek immediate medical attention.